millikan oil drop experiment
Today we are discussing JJ. Millikans Oil Drop Experiment was an experiment performed by the American physicist Robert A.
 |
| Learn Millikan S Oil Drop Experiment In 2 Minutes |
At the time of Millikans work it was not agreed.
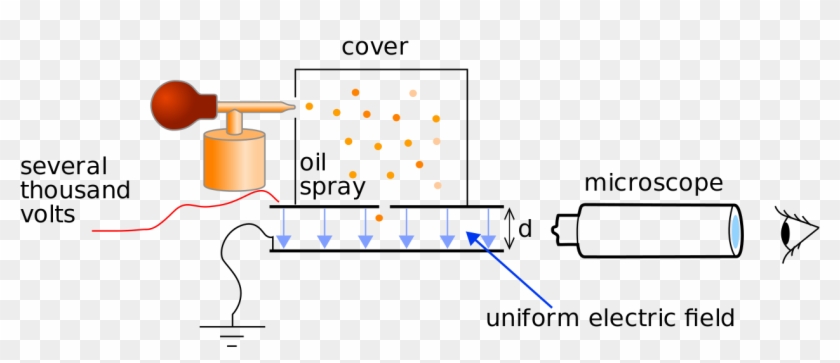
. Millikan Oil-drop Experiment P310 R. This video covers the famous Millikan experiment determining the charge of an electron. Millikan believed that there was a smallest unit of charge and he set. With the help of.
Robert Millikans famous oil drop experiment reported in August 1913 elegantly measured the fundamental unit of electric charge. Oil droplets were sprayed in a fine mist from an atomizer becoming. Millikan Oil Drop Experiment is one of the most popular experiments as it was the first-ever experiment that gave us the direct measurement of the charge of a single electron. Your professor is Robert Millikan.
Robert Millikan was awarded the Nobel Prize in physics in 1923 for this brilliant experiment. What did Millikan oil drop experiment prove. Here are the steps Millikan followed in his successful experiment for which he was awarded the Nobel Prize in 1923. The Oil Drop Experiment In 1909 Robert Millikan and Harvey Fletcher conducted the oil drop experiment to determine the charge of an electron.
Millikans oil drop experiment proved that electric charge is quantized. The experiment was performed by spraying a mist of oil droplets into a chamber above the metal. Silas Millikan Roberts father was a college-educated man and staunchly maintained that the curve ball was simply an optical illusion but young Millikan knew better. Procedure of Millikans Oil Drop Experiment The electric field is then established between the two plates causing the charged oil droplets motion to be impacted.
The Millikan Oil-Drop Experiment HISTORY The year is 1911 and you are taking a physics course. They suspended tiny charged droplets of. A ne spray of oil is injected in the region between the hori-zontal capacitor plates that are connected to an external power supply. Thomsons discovery of electrons and how Robert Millikan with the help of Harvey Fletcher used that knowledge to conduct the O.
Patterson INTRODUCTION The electric charge carried by a particle may be calculated by measuring the force experienced by the particle in an electric. The oil is now being drawn. Robert Millikans oil drop experiment measured the charge of the electron. Millikan and Harvey Fletcher in 1909 to measure the electric charge of a single electron.
In this experiment you will experimentally determine the quantum nature of charge. This was found to be roughly -16 x 10 -19 coulombs by Robert Millikan in the oil-drop experiment Millikan 1911Millikan 1917 Franklin 1997. The experiment a great improvement over previous. A Millikan oil-drop apparatus is shown in Fig.
Done in collaboration with Simon Crook Crooked Science and Tom Gor. Professor Millikan has you and your classmates doing a lab.
 |
| Millikan Oil Drop Experiment Poster By Jose Antonio Pe As Fine Art America |
 |
| Millikan S Oil Drop Experiment Determination Of Charge Of An Electron |
/Simplified_scheme_of_Millikans_oil-drop_experiment.svg-637e1100c6bc49a08b6ca32d8e8feed8.png) |
| The Millikan Oil Drop Chemistry Experiment |
 |
| Physics Experiment Leai 42 Apparatus Of Millikan S Experiment Advanced Model |
 |
| The Milikan Oil Drop Experiment |
Posting Komentar untuk "millikan oil drop experiment"